Laboratory of fluoro-organic compounds
Main research trends
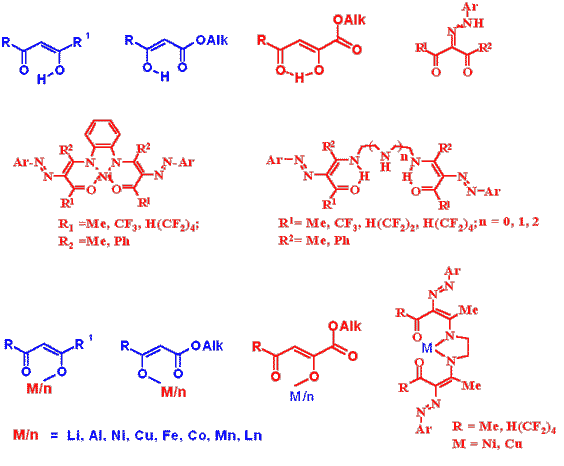
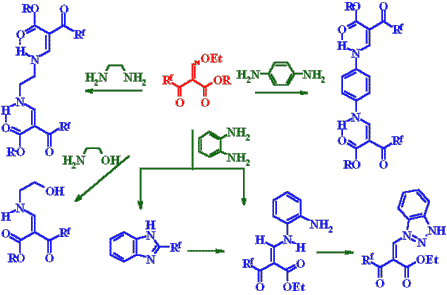
The laboratory is working on the development of synthesis methods and transformation paths of fluorine-containing heterocycles such as pyrazoles, isoxazoles, imydazoles, piperazines, pyrimidines, azoloazines, quinoxalines, chromones, coumarins, quinolones, cinnolones and others. Fluorine-containing -keto esters, -diketones, -oxo esters, -diketones, ,-dioxo esters, ,'-tricarbonyl compounds, fluoroketones, fluoroolefines, fluorinated a-epoxides have been used as precursors for the synthesis of fluoroheterocycles. We study also paths of remarking and utilization of polychlorodiphenyls that are strong ecotoxicants. Polychlorodiphenyls (more 500 thousands of ton) were produced in the former USSR. Technologies of their remarking and utilization are absent in Russia. We elaborate synthetic procedures for fluorination of polychlorodiphenyls for preparation of dielectrics. We investigate also their reaction with oligoethyleneglycoles to obtain the safe compounds, which may use as lubricants. We would like to offer you the cooperation between our laboratories in the field of Chemistry developed by you. If you are interested in the cooperation with us we shall be glad to send you further information and consider your propositions to discuss our joint work.
For example:
1. Target in Heterocyclic Systems, 2001, V. 5, N, P.419
2. Heterocycles, 2000, V.52, N3, 1411-1434.
3. Russian Chemical Rev., 2001, V.70, N11, p.921-938
4. Russian Chemical Rev., 1999, V.68, P.3, p.203
5. Russian Chem. Rev., 1985, V.54, p.1185
6. Russian Chem. Rev., 1982, V.51, p.736.
7. Russian Chem. Rev., 1981, V.50, p.180.
8. J. Fluorine Chem., 2003,V.120, N1, p.41
9. J. Fluorine Chem. 2002, V. 117, N 1, pp. 1-7.
10. J. Fluorine Chem., 2000,V.103, N1, p.3-12.
11. J. Fluorine Chem., 2000,V.104, N2, p.155-165.
12. J. Fluorine Chem., 2000,V.103, N1, p.17-23.
13. J. Fluorine Chem., 1999,V.94, p.83-90.
14. J. Fluorine Chem., 1999,V.94, p.11-13.
15. J. Fluorine Chem., 1998,V.92, p.101-108.
16. J. Fluorine Chem., 1998,V.87, p.49-55;
17. J. Fluorine Chem., 1997,V.84, p.107-111;
18. J. Fluorine Chem., 1995,V.74, p.15-19;
19. J. Fluorine Chem., 1994,V.69, p.119-126;
20. J. Fluorine Chem., 1994,V.69, p.25-29;
21. J. Fluorine Chem., 1993,V.65, p.37-41;
22. J. Fluorine Chem., 1992,V.56, p.325-334.
The laboratory is working on the development of synthesis methods and transformation paths of fluorine-containing heterocycles such as pyrazoles, isoxazoles, imydazoles, piperazines, pyrimidines, azoloazines, quinoxalines, chromones, coumarins, quinolones, cinnolones and others. Fluorine-containing -keto esters, -diketones, -oxo esters, -diketones, ,-dioxo esters, ,'-tricarbonyl compounds, fluoroketones, fluoroolefines, fluorinated a-epoxides have been used as precursors for the synthesis of fluoroheterocycles. We study also paths of remarking and utilization of polychlorodiphenyls that are strong ecotoxicants. Polychlorodiphenyls (more 500 thousands of ton) were produced in the former USSR. Technologies of their remarking and utilization are absent in Russia. We elaborate synthetic procedures for fluorination of polychlorodiphenyls for preparation of dielectrics. We investigate also their reaction with oligoethyleneglycoles to obtain the safe compounds, which may use as lubricants. We would like to offer you the cooperation between our laboratories in the field of Chemistry developed by you. If you are interested in the cooperation with us we shall be glad to send you further information and consider your propositions to discuss our joint work.
For example:
1. Target in Heterocyclic Systems, 2001, V. 5, N, P.419
2. Heterocycles, 2000, V.52, N3, 1411-1434.
3. Russian Chemical Rev., 2001, V.70, N11, p.921-938
4. Russian Chemical Rev., 1999, V.68, P.3, p.203
5. Russian Chem. Rev., 1985, V.54, p.1185
6. Russian Chem. Rev., 1982, V.51, p.736.
7. Russian Chem. Rev., 1981, V.50, p.180.
8. J. Fluorine Chem., 2003,V.120, N1, p.41
9. J. Fluorine Chem. 2002, V. 117, N 1, pp. 1-7.
10. J. Fluorine Chem., 2000,V.103, N1, p.3-12.
11. J. Fluorine Chem., 2000,V.104, N2, p.155-165.
12. J. Fluorine Chem., 2000,V.103, N1, p.17-23.
13. J. Fluorine Chem., 1999,V.94, p.83-90.
14. J. Fluorine Chem., 1999,V.94, p.11-13.
15. J. Fluorine Chem., 1998,V.92, p.101-108.
16. J. Fluorine Chem., 1998,V.87, p.49-55;
17. J. Fluorine Chem., 1997,V.84, p.107-111;
18. J. Fluorine Chem., 1995,V.74, p.15-19;
19. J. Fluorine Chem., 1994,V.69, p.119-126;
20. J. Fluorine Chem., 1994,V.69, p.25-29;
21. J. Fluorine Chem., 1993,V.65, p.37-41;
22. J. Fluorine Chem., 1992,V.56, p.325-334.




 Head of the Laboratory
Head of the Laboratory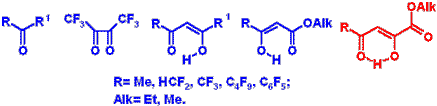









 to the page top
to the page top